Reagent in peptide synthesis, has also been introduced into solid-phase synthesis.
CAS: 26198-19-6
Synonym: 6-Chloro-1-hydroxybenzotriazole dihydrate
Solutions: Luxembourg Bio Technologies can provide various solutions of 6Cl-HOBt, per demand (15% in NMP etc.).
Properties
Purity
≥99.0%
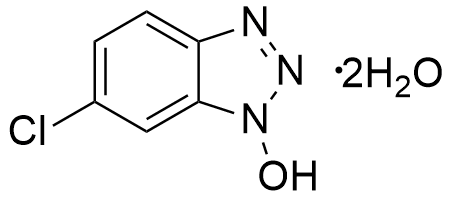
Molecular Formula
C6H4ClN3O·2H2O
Molecular Weight
205.60 [gr/mol]
Appearance
White to off-white crystalline powder
Storage Conditions
Store in a cool and dry place
Applications
- 6-Cl-HOBt increases the efficiency of carbodiimode mediated reactions by generating an active ester that prone for Amidation.
- Moreover, the process suppresses the formation of N-acylurea, which is a stable inert form of the incoming acid. The reaction, which is irreversible and consumes starting acid without generating peptide.
- The beneficial effect of 6-Cl-HOBt is attributed to its capacity to protonate O-acylisourea, thus preventing the intramolecular reaction from occurring and shifting the reaction to form the active ester, end thereby decreasing the degree of racemization in numerous cases.
- 6-Cl-HOBt has been introduced into solid-phase peptide synthesis. This additive is a good compromise between HOAt and HOBt in terms of reactivity and price
Studies
- Assessment of 6Cl-HOBt-based coupling reagents in solid-phase cyclopeptide synthesis
G. Sabatino; M. C. Alcaro; M. C. Pozo-Carrero; M. Chelli; P. Rovero; A. M. Papini Peptide Revolution: Genomics, Proteomics & Therapeutics, 2004, 49-50.
Read Article - Developments in Peptide and Amide Synthesis
F. Albericio Current Opinion in Chemical Biology 2004, 8:211–221.
Read Article - Synthesis and Aminolysis of N,N-Diethyl Carbamic Ester of HOBt Derivatives
S. N. Khattab, S. Y. Hassan, E. A. Hamed, F. Albericio, and A. El-Faham Bull. Korean Chem. Soc. 2009, Vol. 30, No. 12.
Read Article