PyOxim® is a sustainable and highly efficient coupling reagent used in fragment condensation and difficult coupling assays.
CAS: 153433-21-7
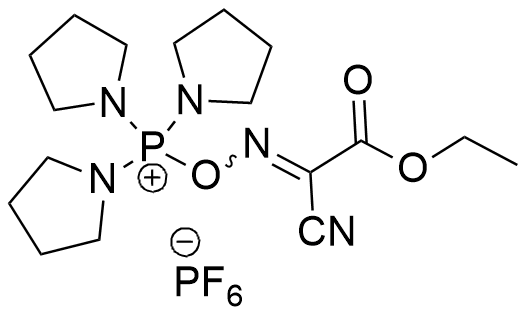
Synonym:1-Cyano-2-ethoxy-2-oxoethylideneaminooxy-tris-pyrrolidino-phosphonium hexafluorophosphate; O-[(1-cyano-2-ethoxy-2-oxoethylidene)amino]-oxytri(pyrrolidin-1-yl) phosphonium hexafluorophosphate
Properties
Purity
≥99.0%
Molecular Formula
C17H29F6N5O3P2
Molecular Weight
527.38 [gr/mol]
Appearance
White crystalline powder
Storage Conditions
Store in a cool and dry place (2-8oC)
Applications
- Effective in difficult cuoplings in SPPS comparable to HATU.
- Highly effective with low racemization in fragment condensation assays.
- It has excellent solubility in DMF and is stable in solution under an inert atmosphere for two days.
- PyOxim® shows higher stability in acetone and DMF than all benzotriazole counterparts, consequently standing out as promising choice for cyclization.
- PyOxim® is safer to handle and less allergenic than HOBt-based coupling reagents, and it is not explosive under normal operating conditions.
Studies
- PyOxP and PyOxB: the Oxyma-based novel family of phosphonium salts
Ramon Subir´os-Funosas, Ayman El-Faham and Fernando Albericio Org. Biomol. Chem., 2010, 8, 3665–3673.
Read Article - Fast conventional Fmoc solid-phase peptide synthesis: a comparative study of different activators
Christina Ann Chantell, Michael Abayomi Onaiyekan and Mahendra Menakuru J. Pept. Sci. 2012; 18: 88–91.
Read Article - Kilogram-Scale GMP Manufacture of Tirzepatide Using a Hybrid SPPS/LPPS Approach with Continuous Manufacturing
Reactivity and Racemization
Solid-Phase Fragment Condensation of Z-Gly-Gly-Val-OH + H-Pro-Gly-Gly-NH-Resin (1) in 2eq. DIEA
| Coupling Reagent | DL (%) |
| PyAOP | 33.7 |
| PyBOP | 51.0 |
| PyClock | 43.1 |
| PyOxim | 22.2 |
Solution Cyclization of H-Ala-Ala-MeAla-Ala-Ala-OH (1)
| Coupling Reagent | Cyclic(%) | Linear (%) | Linear Dimer (%) |
| PyAOP | 54 | 10 | 36 |
| PyBOP | 43 | 28 | 29 |
| PyClock | 61 | 15 | 24 |
| PyOxim | 70 | 10 | 20 |
Manufacture of Tirzepatide (GIP/GLP-1 dual agonist). Fragment coupling. (3)
| Fragments coupling | reagent | solvent | Base | Coupling time/temp | Yield after purification % |
| AA 22-20 + AA 30-39 | PyOxim | DMSO:ACN | DIEA | 1h/200C | 75-80 |
| AA22-39 + AA 15-21 | PyOxim | DMSO: ACN | DIEA | 3h/200C | 68-75 |
| AA 15-39+ AA1-14 | HATU | DMF:ACN | DIEA | 3h/00C | 95-100 |
Properties
Green chemistry
Pyoxim is a much greener coupling reagent that PyBOP, hazardous and mutagenic HOBt is not used in production. It is also safer for the user than uronium(guanidinium) coupling reagents because it does not produce toxic urea waste and is not a sensitizer.
Stability
PyOxim solid is stable at 540C for at least two weeks. Stable for at least 24 months if stored at RT and keep away from light.
Shows superior to PyBOP stability in a solvent.
| 0.25 M solutions of coupling reagents in DMF | 0 days | 1 day | 2 days |
| PyOxim | 99 | 82 | 47 |
| PyBOP | 99 | 73 | 34 |
Solubility
| Coupling Reagent | DMF | DCM |
| PyAOP | 260mg/0.25ml (1.99M) | 230mg/0.25ml (1.79M) |
| PyBOP | 200mg/0.25ml (1.54M) | 160mg/0.25ml (1.23M) |
| PyClock | 200mg/0.25ml (1.44M) | 100mg/0.25ml (0.72M) |
| PyOxim | 340mg/0.25ml (2.58M) | 260mg/0.25ml (1.97M) |
Flamability and Expolsivity hazard
According to test results, PyOxim should not be regulated as a Class 1 explosive and Class 4.1 flammable.
Packing
We are offering several packing options that can be tailored to the customer’s needs.
-Various-sized HDPE stacking containers with Black antistatic PE liner.
-DoverPac® provides a high level of powder containment during loading and offloading operation.