COMU® features optimal properties as a peptide coupling reagent. In addition to its high and fast coupling efficiency, it shows very low or non-existent tendencies for racemization. COMU® is a registered patent of Luxembourg Bio Technologies (No. 101784522/8,524,898).
COMU® is a registered patent of Luxembourg Bio Technologies Ltd., and we are the exclusive manufacturers.
CAS: 1075198-30-9
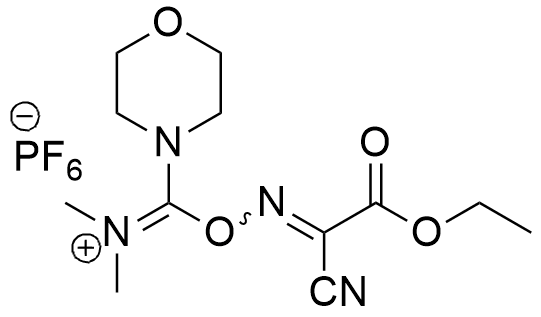
Synonym: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
Properties
Purity
≥98.0%
Molecular Formula
C12H19F6N4O4P
Molecular Weight
428.27 [gr/mol]
Appearance
White to off-white crystalline powder
Storage Conditions
Store in a cool and dry place (2-8oC)
Applications
- Epimerization during fragment coupling appears to be lessened with COMU® than with HOBt or HATU.
- COMU® is fully compatible with microwave-assisted peptide synthesis.
- COMU® is very soluble in most commonly employed peptide coupling solvents, such as DMF or NMP, which makes it ideally suited for solid-phase peptide synthesis.
- It is equally attractive for solution-phase synthesis since by-products formed by COMU® are water-soluble and can be separated by simple extraction.
- A color change during the reaction allows visual or colorimetric reaction monitoring.
- COMU® can be used with nearly identical protocols that apply for common coupling reagents such as HBTU, TBTU, PyBOP, or HATU.
- It is considered the greener coupling reagent.
- It is used in some Canadian schools.
Studies
- A Comparative Study of Amide-Bond Forming Reagents in Aqueous Media – Substrate Scope and Reagent Compatibility
Matthew Badland, Robert Crook, Bastien Delayre, Steven J. Fussell, Iain Gladwell, Michael Hawksworth, Roger M. Howard, Robert Walton, Gerald A. Weisenburger, Tetrahedron Letters, 58, 4391–4394 (2017).
Read Article - Tandem Deprotection/Coupling For Peptide Synthesis in Water at Room Temperature
Margery Cortes-Clerget, Jean-Yves Berthon, Isabelle Krolikiewicz-Renimel, Laurent Chaisemartin, and Bruce H. Lipshutz, Green Chem., 19, 4263 (2017).
Read Article - Total Synthesis of Nannocystin Ax
Caroline Poock and Markus Kalesse, Org. Lett., 19, 4536−4539 (2017).
Read Article - Phenylglycine Racemization in Fmoc-Based Solid-Phase Peptide Synthesis: Stereochemical Stability is Achieved by Choice of Reaction Conditions
Chen Liang, Mira A.M. Behnam, Tom R. Sundermann, Christian D. Klein, Tetrahedron Letters, 58, 2325–2329 (2017).
Read Article - Comparison of Various Coupling Reagents in Solid-Phase aza-Peptide Synthesis
Meeli Arujõe, Anu Ploom, Anton Mastitski, Jaak Järv Tetrahedron Letters, 58, 3421–3425 (2017).
Read Article - COMU®: A Safer and More Effective Replacement for Benzotriazole-Based Uronium Coupling Reagents
A. El-Faham, R. S. Funosas, R. Prohens, and F. Albericio, Chem. Eur. J., 15, 9404–9416 (2009).
Read Article - COMU®: A Third Generation of Uronium-Type Coupling Reagents
A. El-Faham and F. Albericio J. Pept. Sci.,16, 6–9 (2010).
Read Article - Microwave Heating in Solid-Phase Peptide Synthesis
Tofteng, L. Malik, S. L. Pedersen, K. K. Sørensen, K. J. Jensen, Chem. Soc. Rev., 41, 1826–1844 (2012).
Read Article - Efficient and Controllably Selective Preparation of Esters Using Uronium-Based Coupling Agents
J. K. Twibanire and T. B. Grindley, Org. Lett., 13, 12, 2988–2991 (2011).
Read Article - Tandem Deprotection/Coupling for Peptide Synthesis in Water at Room Temperature
Margery Cortes-Clerget, Jean-Yves Berthon, Isabelle Krolikiewicz-Renimel, Laurent Chaisemartin, and Bruce H. Lipshutz, Green Chem., 19, 4263 (2017).
Read Article
Posters
- A Non-Explosive Replacement for Benzotriazole-Based Coupling Reagents
Ayman El-Faham, Ramon Subirós-Funosas, Fernando Albericio
View Poster