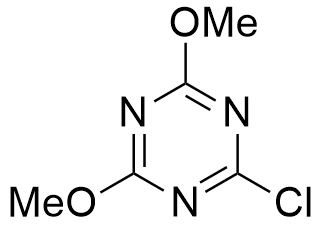
The use of CDMT as a coupling agent has been investigated fairly extensively, and it demonstrates several advantages over other coupling agents. It is a stable, crystalline compound, with good solubility in organic solvents, and is commercially available in large quantities.
CAS: 3140-73-6
Synonym: 2-Chloro-4,6-dimethoxy-1,3,5-triazine
Properties
Purity
≥98.0%
Molecular Formula
C5H6ClN3O2
Molecular Weight
175.57 [gr/mol]
Appearance
White to off-white crystalline powder
Storage Conditions
Store in a cool and dry place (2-8oC)
Applications
- The standard method for making amides using CDMT is to activate the acid using CDMT and a base such as N-methylmorpholine. This generates an active ester which is subsequently reacted with the amine coupling partner in the same pot. The reaction typically proceeds to completion in a matter of 8–14 h. This method is effective for the formation of a variety of compounds, including esters and Weinreb amides. Workup of the products is typically afforded by extraction with dilute acid, as the CDMT and its triazine by-products are typically weakly basic and easily removed extractively.
- When the product is a nonwater soluble solid, the reaction can be run in acetonitrile and many of the products isolated simply by adding water and recovering the precipitate.
- CDMT is a reagent which has been used mainly in SPPS. In the presence of N-methylmorpholine (NMM) as base, the reagent gives low levels of racemization.
- Numerous oligopeptides of 3-heteroaryloamino-2,3-dehydroalanine have been obtained using CDMTas the coupling reagent.
- In the pure state CDMT is stable almost indefinitely (20 years in the author’s laboratory) without any traces of decomposition (Kamin´ ski, 1996).
Studies
- Recent development in peptide coupling reagents
T. I. Al-Warhi, H. M.A. Al-Hazimi, and A. El-Faham Journal of Saudi Chemical Society, 2012, 16, 97–116.
Read Article - A novel generation of coupling reagents. Enantiodifferentiating coupling reagents prepared in situ from 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) and chiral tertiary amines
Z. J. Kamiński, B. Kolesińska, J. E. Kamińska, and J. Góra J. Org. Chem., 2001, 66, 6276-6281.
Read Article - New observations on peptide bond formation using CDMT
C. E. Garrett, X.g Jiang, K. Prasad, and O. Repic Tetrahedron Lett., 2002, 43, 4161–4165.
Read Article - CDMT at e-EROS: Encyclopedia of Reagents for Organic Synthesis.
-
Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing
Jeffrey B. Sperry*, Christopher J. Minteer, JingYa Tao, Rebecca Johnson, Remzi Duzguner, Michael Hawksworth, Samantha Oke, Paul F. Richardson, Richard Barnhart, David R. Bill, Robert A. Giusto, and John D. Weaver III
Org. Process Res. Dev. 2018, 22, 9, 1262–1275